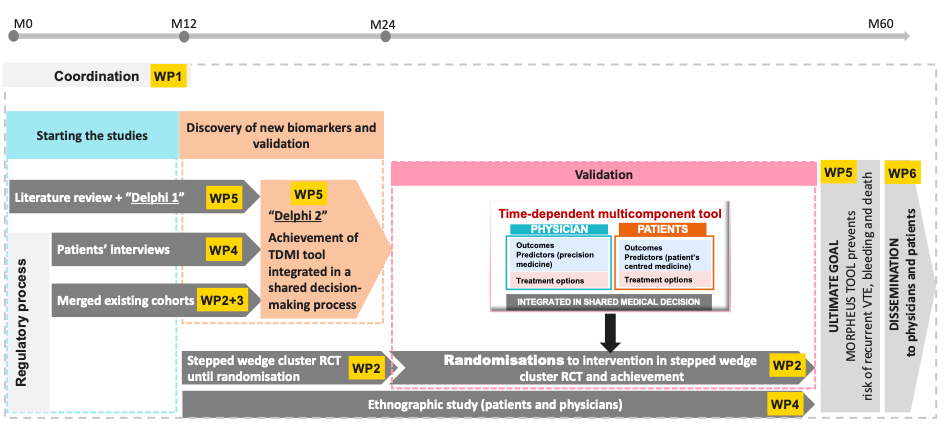
MORPHEUS will be organised through 6 work packages over a five-year timescale
WP1: coordination for Morpheus achievement goals
OBJECTIVES
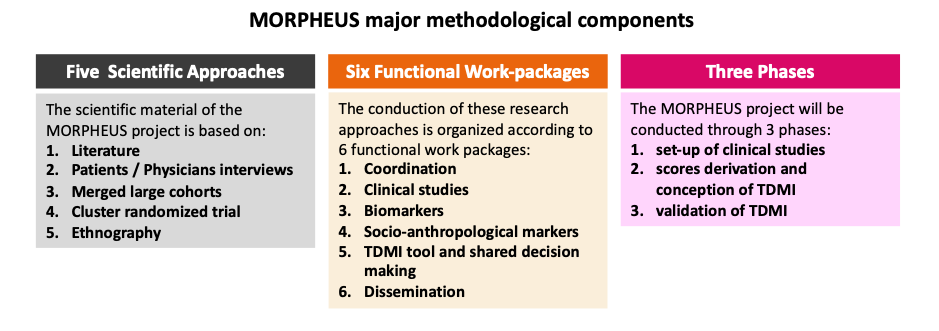
- MORPHEUS is conducted through 3 phases, during which 5 scientific approaches will be conducted by 6 work-packages during a 5-year period (1st dec 2023 to 30 Nov 2028).
- Coordination of MORPHEUS requires therefore strong governance and an adequate management structure to ensure the most efficient collaboration between all partners, to monitor project’s progress towards its objectives, to anticipate possible hurdles, to solve them and to ensure fulfilment of the highest ethical standards by all partners.
METHODS
- Step 1: to set up the five scientific approaches ((literature review/Delphi1, patients’ and physicians’ interviews [anthropology], merged cohorts [n=14 studies], step wedged cluster randomized “ETHER” study, ethnography) that are necessary to achieve the project (coordination by WP1). All these scientific approaches will be conducted by six functional work-packages that will closely work together.
- Step 2: to develop the time-dependent multicomponent intervention (TDMI) tool integrated in a shared decision-making process. This tool should be achieved at month 24, before starting randomisation of clinical centres in the cluster RCT “ETHER study”. This TDMI tool implementation will be based on the first three scientific approaches described above (literature review, patients’ and physicians’ interviews ([anthropology] and merged cohorts).
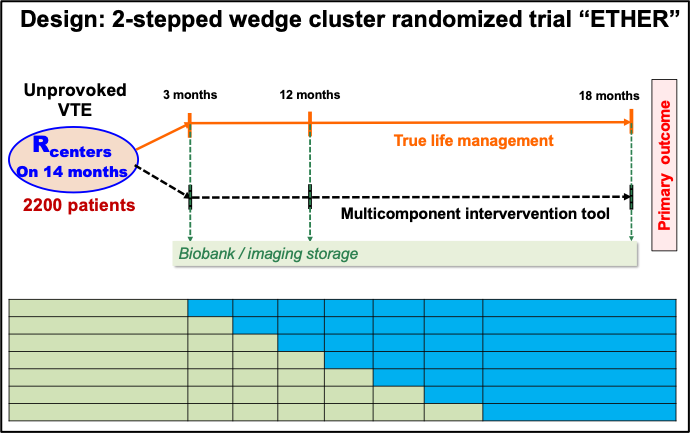
- Step 3: to validate the beneficial impact of the TDMI integrated in a shared decision-making tool on the primary composite outcome. We will achieve this goal via a 2-stepped wedge cluster randomized “ERTHER” trial designed to demonstrate the effectiveness of the TDMI tool (leading to a shared decision-making) on the main primary composite outcome.

WP 2: Derivation and validation studies
OBJECTIVES
- The objectives of WP2 will be achieved three objectives through 3 sub-work-packages:
- WP2a: Definition and assessment of studies outcomes
- WP2b: Merged cohort coordination
- Cluster RCT “ETHER” conduction
METHODS
- Definition and assessment of studies outcomes with the Trial Steering Committee (TSC) and the independent Clinical Events Committee (iCEC)
- Merging of existing cohorts (clinical, biological, imaging data)
- Control or obtain regulatory and ethical approvals for the merged cohorts
- Selection of variables of cohorts to be merged and creation of database
- Selection and realisation of additional biological analyses to be perform
- Selection and realisation of additional analyses on imaging
- Conduction of the cluster RCT “ETHER” for TDMI tool validation
- Clinical study European regulatory approvals
- Patients recruitment and follow-up
- Full database for study data analysis
WP3: Biomarkers identification
OBJECTIVES
- The main objective is to identify relevant and accurate time-dependant biomarkers based on merged cohorts that will allow derivation of predictive scores by WP5.
- The objectives of WP3 will be achieved by 3 sub-work-packages:
- WP3a: Identification of biological biomarkers
- WP3b: Identification of biomarkers on pharmacology and pharmacodynamic
- WP3c: Identification of imaging biomarkers
METHODS
- Identification of biological biomarkers: perform measurements for current metabolite, miRNA and RNAs, proteomics, genetics
- Discovery new biomarkers
- Identification of biomarkers for pharmacology and pharmacodynamic
- Identification of imaging biomarkers
WP4: Sociological and anthropological approaches
OBJECTIVES
- Explore in detail and understand what influences current practice in anticoagulant management of patients with unprovoked VTE, their careers, and healthcare professionals.
- Identify patients’ experience and perceptions regarding risks (recurrent VTE, bleeding, death) and other parameters linked to quality of life and treatment and the potential barriers and facilitators to stop or to extend anticoagulation in patients with unprovoked VTE, using qualitative research.
- Evaluate practical solutions to enhance acceptability and satisfaction of patients and physicians with TDMI and its impact on shared decision-making process in the setting of unprovoked VTE
METHODS
- Prepare the qualitative studies
- To conduct qualitative interviews to inform the multicomponent tool development (“Anthropo-Morpheus” study)
- To study the MORPHEUS decision-support tool during the design phase (“Ethno-Morpheus”study)
- To provide a follow-up of the MORPHEUS decision support tool during the cluster RCT
WP5: Multicomponent intervention tool conception and Methodology and statistical analysis
OBJECTIVES
- The main objective is to build the TDMI and to define the shared decision-making process that will be validated in the cluster RCT “ETHER”
- To reach these objectives, the WP is structured in two sub work-packages:
- WP5a: Building the TDMI based on the literature review
- WP5b: Methodology and statistical analyses
METHODS
- To identify most discriminant biomarkers from literature review (Delphi 1)
- Derivation of scores from merged cohort
- TDMI conception (Delphi 2)
- Define the shared decision-making process
- To perform data analysis of the cluster RCT (“ETHER study”)
WP6: Dissemination
OBJECTIVES
- To ensure the dissemination of the results
- To perform the design of the score calculator and app, and commercialization plan of the end-product
METHODS
- Public relations activities: graphical chart, public internet website, presentation leaflets and posters, prepare the newsletters, coordinate press release
- Dissemination of the results of the trial and the economic evaluation to the scientific community and stakeholders
- Design and development of a final version of multicomponent intervention tool (app)
- Commercialization plan
